Research Interests
Small Molecule Activation and Catalyltic Conversion
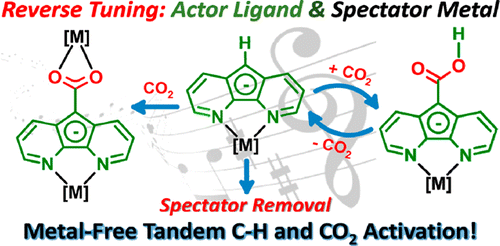
The efficient conversion of naturally abundant small molecules into value-added products is an elegant way of utilizing natural resources, exemplified by numerous enzymatic processes developed by nature.Our group is particularly interested in the activation and catalytic conversion of atmospheric small molecules such as N2, O2, and CO2.
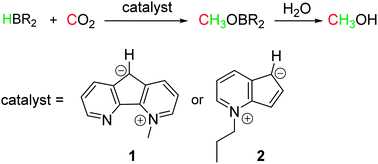
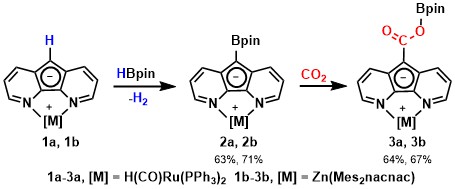
J. Am. Chem. Soc. 2013, 135, 16175-16183.
Chem. Commun. 2015, 51, 11293-11296.
Chem. Commun. 2016, 52, 4148-4151.
Inorg. Chem. 2017, 56, 11956-11970.
Chem. Commun. 2017, 53, 11390-11398.
Base Metal Chemistry
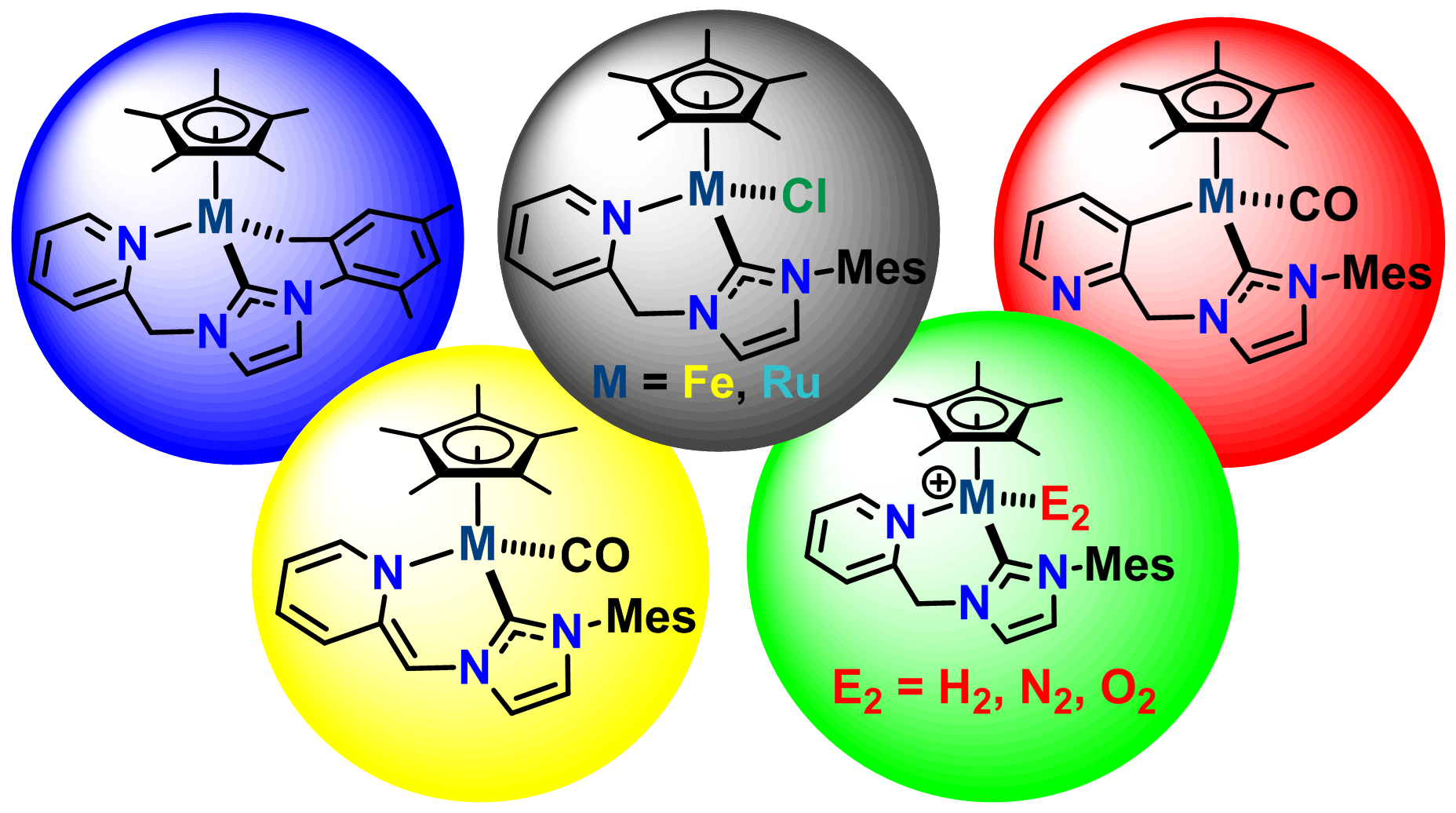
Replacing noble metal-based homogeneous catalysts with base metal counterparts is an attractive approach. In addition to the lower cost, base metal catalysts are more environmentally friendly than their noble metal counterparts. Moreover, the use of high-spin metal centers can potentially result in lower activation barriers and can invoke one-electron processes that are less prevalent in noble metal chemistry.
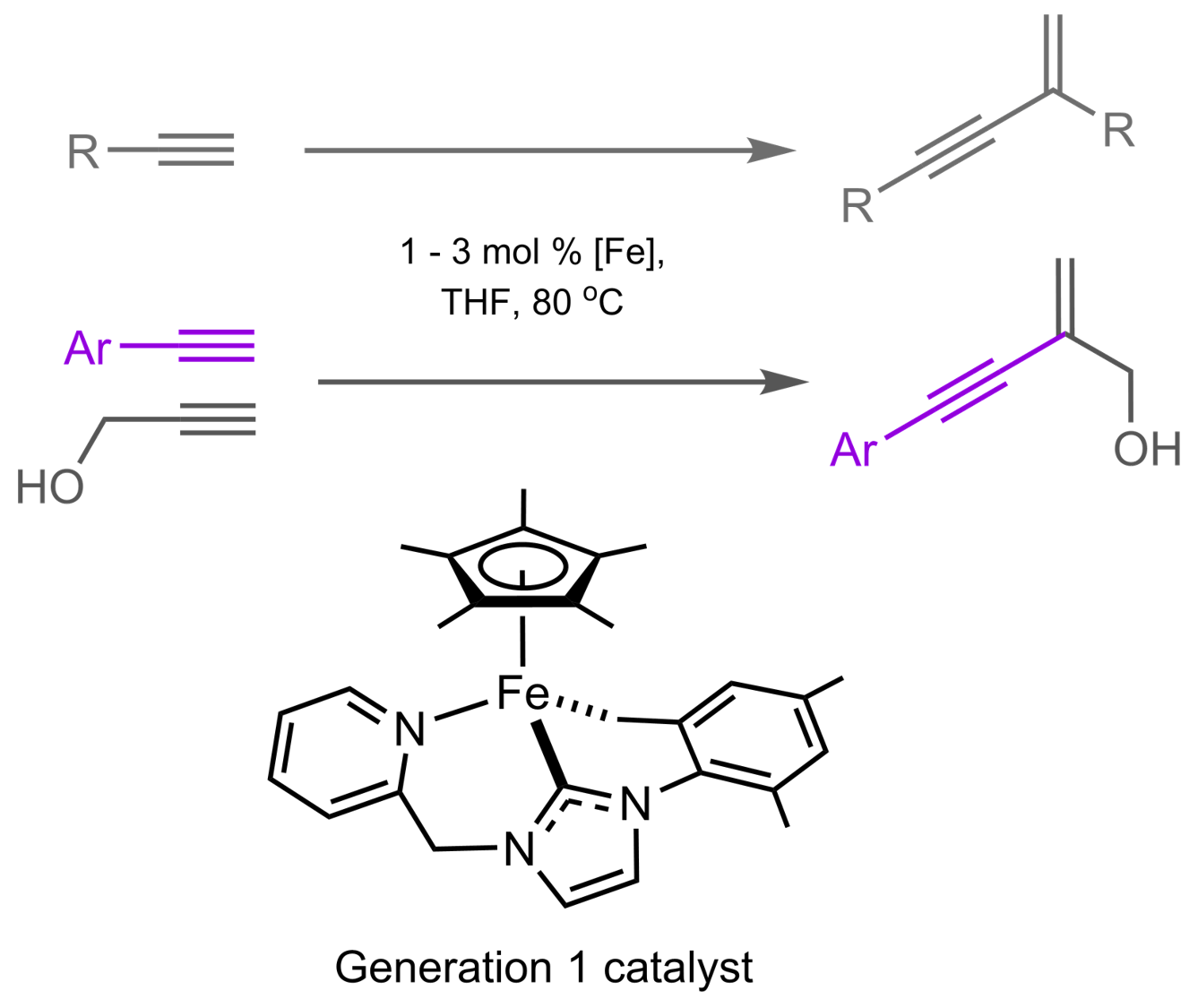
Angew. Chem. Int. Ed. 2017, 56, 6317-6320.
J. Am. Chem. Soc. 2018, 140, DOI: 10.1021/jacs.7b13097
Metal-Organic Frameworks (MOFs)
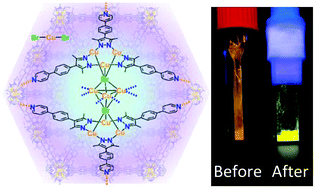
We are also researching metal organic frameworks (MOFs) with a focus on sensing and gas adsorption. We have developed MOFs with sensing capabilities toward volatile oragnics (VOCs) and with selective gas adsorption properties.